Magnesium And Zinc Chloride Reaction . Other metals, like copper, resist reacting to even corrosive acids. Web these definitions are useful in reactions that do not involve oxygen. Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web the following equation describes a reaction between magnesium and zinc chloride: For example, magnesium reacts with chlorine to form. Zncl2 aqueous plus mg solid reacts to. Acid + metal → salt + hydrogen. Web one example of a displacement reaction is the reaction of magnesium dipped in. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Some metals, like sodium, react simply by being placed in water. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.
from www.numerade.com
Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Web these definitions are useful in reactions that do not involve oxygen. Web one example of a displacement reaction is the reaction of magnesium dipped in. Some metals, like sodium, react simply by being placed in water. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Web the following equation describes a reaction between magnesium and zinc chloride: Zncl2 aqueous plus mg solid reacts to. Other metals, like copper, resist reacting to even corrosive acids. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. For example, magnesium reacts with chlorine to form.
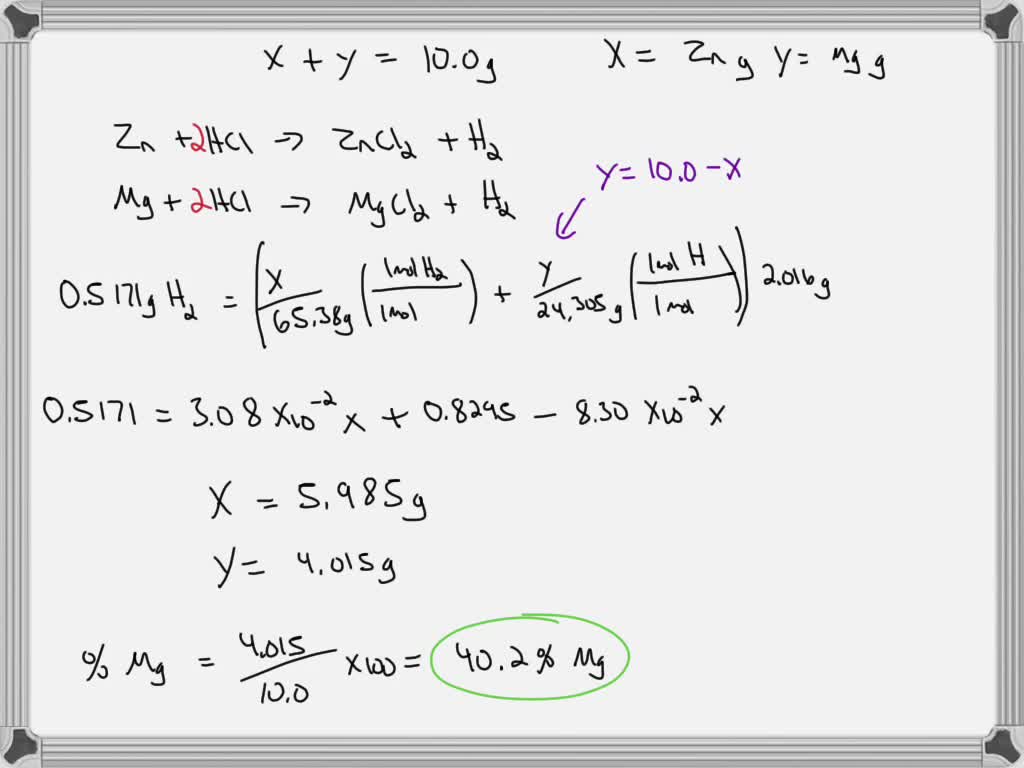
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with
Magnesium And Zinc Chloride Reaction Acid + metal → salt + hydrogen. Web one example of a displacement reaction is the reaction of magnesium dipped in. Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Web these definitions are useful in reactions that do not involve oxygen. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Other metals, like copper, resist reacting to even corrosive acids. Web the following equation describes a reaction between magnesium and zinc chloride: Some metals, like sodium, react simply by being placed in water. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Zncl2 aqueous plus mg solid reacts to. Acid + metal → salt + hydrogen. For example, magnesium reacts with chlorine to form.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium And Zinc Chloride Reaction For example, magnesium reacts with chlorine to form. Web one example of a displacement reaction is the reaction of magnesium dipped in. Zncl2 aqueous plus mg solid reacts to. Some metals, like sodium, react simply by being placed in water. Other metals, like copper, resist reacting to even corrosive acids. Web the experiment reinforces ideas about energy changes during reactions,. Magnesium And Zinc Chloride Reaction.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium And Zinc Chloride Reaction Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. For example, magnesium reacts with chlorine to form. Web the following equation describes a reaction between magnesium and zinc chloride: Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web these definitions. Magnesium And Zinc Chloride Reaction.
From www.istockphoto.com
Magnesium Chloride Formation Illustration Reaction Of Magnesium With Magnesium And Zinc Chloride Reaction Acid + metal → salt + hydrogen. Web one example of a displacement reaction is the reaction of magnesium dipped in. Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zncl2 aqueous plus mg solid reacts. Magnesium And Zinc Chloride Reaction.
From www.numerade.com
SOLVED An aqueous solution of magnesium chloride, MgCl2, is added to Magnesium And Zinc Chloride Reaction For example, magnesium reacts with chlorine to form. Web the following equation describes a reaction between magnesium and zinc chloride: Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Some metals, like sodium, react simply by being placed in water. Acid + metal → salt + hydrogen. Other metals, like copper,. Magnesium And Zinc Chloride Reaction.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Magnesium And Zinc Chloride Reaction Some metals, like sodium, react simply by being placed in water. Web these definitions are useful in reactions that do not involve oxygen. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.. Magnesium And Zinc Chloride Reaction.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium And Zinc Chloride Reaction Some metals, like sodium, react simply by being placed in water. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zncl2 aqueous plus mg solid reacts to. Other metals, like copper, resist reacting to even corrosive acids. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole. Magnesium And Zinc Chloride Reaction.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Magnesium And Zinc Chloride Reaction Web the following equation describes a reaction between magnesium and zinc chloride: Zncl2 aqueous plus mg solid reacts to. Web these definitions are useful in reactions that do not involve oxygen. Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Other metals, like copper, resist reacting to even corrosive acids. Web. Magnesium And Zinc Chloride Reaction.
From www.numerade.com
SOLVED 1. Magnesium and hydrochloric acid react to form magnesium Magnesium And Zinc Chloride Reaction Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Other metals, like copper, resist reacting to even corrosive acids. Web one example of a displacement reaction is the reaction of magnesium dipped in. Web these definitions are useful in reactions that do not involve oxygen. For example, magnesium reacts with chlorine to form. Web. Magnesium And Zinc Chloride Reaction.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Magnesium And Zinc Chloride Reaction For example, magnesium reacts with chlorine to form. Other metals, like copper, resist reacting to even corrosive acids. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Web these definitions are useful in reactions that do not involve oxygen. Web for example, zinc metal reacts. Magnesium And Zinc Chloride Reaction.
From mavink.com
Magnesium And Dilute Hydrochloric Acid Magnesium And Zinc Chloride Reaction Some metals, like sodium, react simply by being placed in water. Acid + metal → salt + hydrogen. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Zncl2 aqueous plus mg solid. Magnesium And Zinc Chloride Reaction.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Magnesium And Zinc Chloride Reaction Acid + metal → salt + hydrogen. Other metals, like copper, resist reacting to even corrosive acids. Some metals, like sodium, react simply by being placed in water. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web one example of a displacement reaction is the reaction. Magnesium And Zinc Chloride Reaction.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Magnesium And Zinc Chloride Reaction Acid + metal → salt + hydrogen. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. For example, magnesium reacts with chlorine to form. Web the following equation describes a. Magnesium And Zinc Chloride Reaction.
From www.slideserve.com
PPT Rate of Reactions PowerPoint Presentation, free download ID6722004 Magnesium And Zinc Chloride Reaction Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Web these definitions are useful in reactions that do not involve oxygen. For example, magnesium reacts with chlorine to form. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Magnesium And Zinc Chloride Reaction.
From www.slideserve.com
PPT Net Ionic in SR PowerPoint Presentation, free download ID5979486 Magnesium And Zinc Chloride Reaction Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Web the following equation describes a reaction between magnesium and zinc chloride: Web these definitions are useful in reactions that do not involve. Magnesium And Zinc Chloride Reaction.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Magnesium And Zinc Chloride Reaction Web these definitions are useful in reactions that do not involve oxygen. Acid + metal → salt + hydrogen. Web the experiment reinforces ideas about energy changes during reactions, the reactivity series of the metals and the chemical behaviour of metals. Some metals, like sodium, react simply by being placed in water. For example, magnesium reacts with chlorine to form.. Magnesium And Zinc Chloride Reaction.
From byjus.com
Write the chemical formulae of the following a Magnesium chloride Magnesium And Zinc Chloride Reaction Zncl2 aqueous plus mg solid reacts to. Web acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. For example, magnesium reacts with chlorine to form. Web the following equation describes a reaction between magnesium and zinc chloride: Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.. Magnesium And Zinc Chloride Reaction.
From www.youtube.com
Type of Reaction for Mg + CuCl2 → MgCl2 + Cu YouTube Magnesium And Zinc Chloride Reaction Zncl2 aqueous plus mg solid reacts to. Other metals, like copper, resist reacting to even corrosive acids. For example, magnesium reacts with chlorine to form. Web zn + mgcl2 = zncl2 + mg is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole. Web these definitions are useful in reactions that do not involve. Magnesium And Zinc Chloride Reaction.
From www.sciencephoto.com
Magnesium reacting with HCl Stock Image A500/0633 Science Photo Magnesium And Zinc Chloride Reaction Web the following equation describes a reaction between magnesium and zinc chloride: Web for example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Some metals, like sodium, react simply by being placed in water. Other metals, like copper, resist reacting to even corrosive acids. Zncl2 aqueous plus mg solid reacts to. Web the experiment reinforces ideas. Magnesium And Zinc Chloride Reaction.